Page 1 of 1
Federal and state anti-discrimination agencies have issued guidance for employers that want to require workers to get a COVID-19 vaccine—but at least one lawsuit has claimed that employers can’t mandate a vaccine that is approved only for emergency use. While this argument might not hold up in court, employers should be aware of the risks associated with a vaccine mandate.
When employees refuse a vaccine, the employer should address their concerns and explain the reasons why the company has adopted a mandatory vaccination policy. An open dialogue and education will be key, as will following FDA updates in this regard and consulting with legal counsel.
There are many reasons why an employee may be unwilling to receive a COVID-19 vaccine, and employers may need to explore reasonable accommodations, particularly with employees who have disability-related and religious objections to being vaccinated.
Distribution of COVID-19 vaccines has been issued under the Food and Drug Administration’s (FDA’s) Emergency Use Authorization (EUA) rather than the FDA’s usual processes. But the FDA has said that the vaccine has met its “rigorous, scientific standards for safety, effectiveness and manufacturing quality” and that “its known and potential benefits clearly outweigh its known and potential risks.”
An employee who recently filed a lawsuit challenging an employer’s vaccine mandate argued that the EUA states that people must have “the option to accept or refuse administration of the [vaccine]” and be informed “of the consequence, if any, of refusing administration of the [vaccine] and of the alternatives to the [vaccine] that are available and of their benefits and risks.”
Although the employee in the case works in the public sector, many employment relationships in the private sector are at-will, which means either the employer or the worker can terminate the employment for any lawful reason. An employer that mandates a vaccine may argue the consequence of refusing a vaccine is being fired.
“Consensus in the legal community has been that, at least in the private sector, employers may require at-will employees to be vaccinated, subject to accommodations that may be required for medical or religious reasons,” said Kevin Troutman, an attorney with Fisher Phillips in Houston, and Richard Meneghello, an attorney with Fisher Phillips in Portland, Ore.
The U.S. Equal Employment Opportunity Commission (EEOC) has issued guidance indicating that employers generally can mandate COVID-19 vaccinations. “The EEOC specifically addressed vaccinations that are authorized or approved by the FDA,” noted Anne-Marie Vercruysse Welch, an attorney with Clark Hill in Birmingham, Mich.
The California Department of Fair Employment and Housing (DFEH) also recently said that the Fair Employment and Housing Act (FEHA) generally allows employers to mandate vaccines that have been approved by the FDA. The DFEH specially noted that the FDA has authorized and recommended three COVID-19 vaccines—all of which have been authorized under an EUA.
But vaccine mandates may still be risky for employers. It is possible that employees who are terminated for refusing to receive a vaccine authorized by the FDA under an EUA could try to pursue claims for wrongful termination in violation of public policy. The viability of such claims will depend on applicable state law regarding a potential public policy exception to at-will employment and how courts—state and federal—construe the EUA wording.
The regulatory framework is still unclear and a number of states are considering legislation that would prohibit employers from requiring employees to receive a COVID-19 vaccine. If these bills become law, the uncertainty regarding the EUA issue will become moot in those states, at least as of the time the laws go into effect.
The EEOC issued guidance stating that employees may be exempt from employer vaccination mandates under the Americans with Disabilities Act (ADA), Title VII of the Civil Rights Act of 1964 (Title VII) and other workplace laws.
California’s guidance noted that the FEHA prohibits employers from discriminating against employees or job applicants based on a protected characteristic—such as age, race or sex—and requires employers to explore reasonable accommodations related to a worker’s disability or sincerely held religious beliefs.
“If an employee has a medical condition or sincerely held religious belief that would prevent them from being able to be vaccinated, their employer must go through the interactive process to determine if a reasonable accommodation is available,” Welch said. She recommended that employers have accommodation forms available to employees to begin the interactive process and document the steps the employer took to attempt to arrive at a reasonable accommodation.
Accommodations could take various forms, depending upon the employee’s job and setting. Employers may offer remote work, change the physical workspace, revise practices or provide a leave of absence. In each situation, the employer must determine whether an accommodation would enable the employee to safely perform the essential functions of their job.
Some employees might refuse to receive a vaccine for reasons that aren’t legally protected, such as a general distrust of vaccines. Employers need to be very thoughtful as they consider whether to mandate vaccines because employers may have to fire a material portion of their workforce who refuse to be vaccinated or allow some employees to ignore a company policy–which can lead to discrimination risks and employee morale issues.
“Most employers are encouraging vaccination rather than requiring it,” Welch observed.
Coburn recommended that employers focus on the following measures to encourage employees to receive a vaccination:
Employers that want to offer incentives should be mindful of wellness program limitations and offer alternative ways for employees who cannot get vaccinated to receive the incentives, Coburn noted.
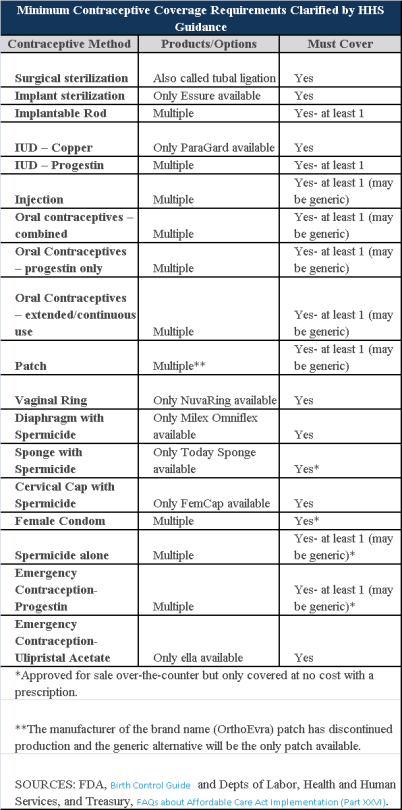
Plans and insurers must cover all 18 contraception methods approved by the U.S. Food and Drug Administration, according to a new set of questions and answers on the Affordable Care Act’s preventive care coverage requirements.
“Reasonable medical management” still may be used to steer members to specific products within those methods of contraception. A plan or insurer may impose cost-sharing on non-preferred items within a given method, as long as at least one form of contraception in each method is covered without cost-sharing.
However, an individual’s physician must be allowed to override the plan’s drug management techniques if the physician finds it medically necessary to cover without cost-sharing an item that a given plan or insurer has classified as non-preferred, according to one of the frequently asked questions from the U.S. Departments of Labor, Health and Human Services and the Treasury.
The ACA mandated all plans and insurers to cover preventive care items, as defined by the Public Health Service Act, without cost-sharing. Eighteen forms of female contraception are included under the preventive care list. The individual FAQs on contraception clarified the following requirements.
The FAQ comes just weeks after reports and news coverage detailed health plan violations of the women coverage provisions of the ACA.
Testing and Dependent Care Answers
In questions separate from contraception, plans and insurers were told they must cover breast cancer susceptibility (BRCA-1 or BRCA-2) testing without cost-sharing. The test identifies whether the woman has genetic mutations that make her more susceptible to BRCA-related breast cancer.
Another question stated that if colonoscopies are performed as preventive screening without cost-sharing, then plans could not impose cost-sharing on the anesthesia component of that service.
Wondering what your employee is smoking in the break room, likely in violation of your “no-smoking” policy? Chances are it is an electronic smoking device, such as an e-cigarette or vaporizer. What should you do about it? Anything?
Many people are familiar with the increasingly popular e-cigarettes and vaporizers, forcing employers to now grapple with the question of whether to permit these devices in the workplace. The answer to this question is constantly changing based on new and revised laws and regulations. It can be difficult to stay aware of this ever-changing issue.
Not A Fad
Electronic smoking devices, particularly vaporizers, are skyrocketing in
popularity. One example of this continued popularity is shown by Oxford
Dictionary’s selection of the word “vape” as the 2014 word of the year. With
around five million Americans currently “vaping” and a $2.5 billion industry
with a 23% rise in sales in 2014, the electronic smoking industry is here to
stay.
There is no indication that growth will slow down any time soon, as sales are projected to surpass $3.5 billion this year and are leaving companies scrambling to meet rising demand.
How They Work
At a basic level, e-cigarettes and vaporizers allow users to consume nicotine
or other substances without smoking tobacco. However, there are noted
differences between e-cigarettes and vaporizers. E-cigarettes are smaller
cigarette-like electronic devices with batteries that heat a cartridge
containing liquid nicotine until it produces an inhalable vapor.
A vaporizer is comparatively larger than an e-cigarette and resembles a large pen. The vaporizer gradually heat the “e-liquid” with warm air, so it tends to last longer than e-cigarettes. Also, the vaporizer is refillable whereas an e-cigarette cartridge must be replaced when spent. To distinguish electronic vapor from smoke, users have coined the term “vaping,” instead of smoking.
The cartridges and refillable liquids contain mostly liquid nicotine and flavoring. However, there are also small amounts of propylene glycol or vegetable glycerin. The e-liquids also come in a wide assortment of flavors, including traditional tobacco as well as kid-friendly ones like gummy bear, snickerdoodle and watermelon mint.
The Controversy.
So what is all the fuss over these devices? To put it simply, there is not a
lot of established data about the health risks. The technology is new, as the
first e-cigarette was marketed in 2002. Manufacturers only introduced
e-cigarettes to the United States in 2007.
Since the products are new, it is not surprising that scientific data is limited. Scientific studies generally conclude that e-cigarettes and vaporizers are less harmful to an individual’s health than traditional tobacco cigarettes. The problem is that no one knows exactly what “less harmful” means.
Due to these uncertainties, there is significant controversy surrounding these devices. Advocates emphasize the value of e-cigarettes and vaporizers as an effective cessation device. Opponents point to the potential health risks. Studies have found that e-cigarette vapor may contain metal particles, carcinogens like formaldehyde, and enough nicotine to cause measurable secondhand exposure. But the long-term effects of e-cigarette vapor and direct exposure to liquid nicotine are unknown.
Federal And State
Prohibitions.
State and local governments were among the first to take action against
e-cigarettes and vaporizers. New Jersey, Utah, and North Dakota placed bans on
the use of the devices in public places and hundreds of cities have followed
suit. For example, Los Angeles, New York, Boston, and Chicago all banned the
use of e-cigarettes in restaurants, bars, nightclubs, and other public spaces.
Several California cities even require vendors to acquire special licenses to
sell e-cigarettes.
The federal government is now weighing in on the debate. The FDA wants to keep a closer eye on e-cigarette manufacturers. The agency is concerned that manufacturers are not required to comply with cleanliness or safety standards. Manufacturers are also currently under no obligation to disclose ingredients. In 2014, the FDA released proposed regulations that would deem tobacco products, including electronic smoking devices, subject to the federal Food, Drug, and Cosmetic Act.
The proposed rule received more than 82,000 comments during the notice and comment period which ended in August of last year, which likely means that it could be some time before the final rules are released. If approved, it would give the FDA the authority to set age restrictions and the power to partake in rigorous scientific review to monitor the ingredients, manufacturing process, and therapeutic claims of e-cigarette companies.
The Pros And Cons
Why is this relevant to you?
Employers must take action and determine their company’s stance on the issue before a supervisor walks into the break room to find the crew puffing on e-cigarettes. While the debate between opposition groups and advocates is just heating up, don’t wait to act. Weigh the pros and cons of allowing electronic smoking devices in the workplace and determine what is best for your company.
There are many factors that employers must take into consideration. Rightly or wrongly, e-cigarettes are perceived as tobacco. The Surgeon General’s office categorizes e-cigarettes as a “tobacco product.” Many state and local governments restrict e-cigarette usage in the same way as tobacco. Some insurance companies already impose a surcharge on e-cigarette users’ premiums, equating e-cigarettes to tobacco.
Employers with nicotine-free hiring policies, particularly those in the healthcare industry, have started to specifically ban e-cigarette users. As a result, these employers are treating users of e-cigarettes and vaporizers the same as users of traditional tobacco products.
Give serious consideration to whether allowing e-cigarette use in your workplace undermines a smoke-free environment. Allowing this practice is likely to cause distractions, may cause questions or concern from customers, and may anger other employees concerned about the secondary impact on their health.
Although the long-term effects of e-cigarettes and vaporizers are currently unknown, the developing consensus is that secondhand vapor contains nicotine or something worse. As a result, secondhand vapor may pose a risk to pregnant women, the elderly, children, and individuals with asthmatic conditions particularly.
Our Advice
We think that the cautious course of action is to treat electronic smoking
devices the same as traditional cigarettes and other tobacco products under
your no-smoking policy. Taking this step and eliminating the risk is simple:
simply amend your employee handbook to include electronic smoking devices,
e-cigarettes, and vaporizers, in the definition of smoking. Employees will then
have to use e-cigarettes outside or offsite, depending on your particular
policy.
By amending your employee handbook, you reduce risk and eliminate distracting behavior. Nonsmoking employees (almost always the majority) will feel comfortable at work and employees who smoke will only be required to comply with an already familiar procedure. Most importantly, employees will not have to worry about the potential harm caused by e-cigarette use or secondhand vapor.
Although amending the handbook may be the easiest answer, remember to follow the proper procedures when amending your handbook. Ensure that any amendments are in compliance with state law, federal law, and especially the National Labor Relations Act. Finally, ensure that all employees receive a copy of the updated handbook, understand the revisions, and acknowledge in writing that they understand the revisions.
Our topic this month covers the Final Rule from HHS and the Exchanges.
Areas discussed include:
Contact us today for more information on this topic.